Axonics Therapy Treatment in San Antonio, TX
What is Axonics Therapy?
Axonics Therapy is a clinically proven solution for treating symptoms of overactive bladder (including urinary urgency incontinence), bowel (fecal) incontinence and urinary retention.1
If you’re considering Axonics Therapy, South Texas Urology Group in San Antonio is here to guide you through your options. This innovative treatment offers long-term relief from overactive bladder and incontinence with a minimally invasive procedure. Contact us today at (210) 267-1709 to schedule your consultation and start your path to improved quality of life.
What are the benefits of Axonics Therapy?
Small Size: Safely delivers therapy with a miniaturized implant
- Long-Term Therapy: Designed to provide long-term symptom relief
- Full-Body MRI: Eligible for full-body MRI under approved conditions
- Clinically Proven: In a clinical study, Axonics Therapy provided sustained safety and symptom relief at 3 years2
How does Axonics Therapy work?

The Evaluation Step: To see if Axonics Therapy is right for you, you will undergo a short period of therapy using a temporary system. The evaluation period allows you to experience the level of symptom relief the therapy may provide before you commit to long-term therapy.
Long-term Therapy: If you and your doctor determine that Axonics Therapy is right for you, you will have an outpatient procedure where the miniaturized Axonics implant is placed just beneath the skin in the upper part of your buttock.
Clinically Proven. Patient Approved.
Backed by clinical studies, Axonics Therapy is clinically proven to help regain bladder and bowel control and deliver clinically meaningful improvement in quality of life.
At 1-Year,
- 93% of patients were satisfied with their therapy1
- 89% of patients experienced a ≥50% reduction in urinary urgency incontinence symptoms1
- <2% of patients reported discomfort at the implant site1
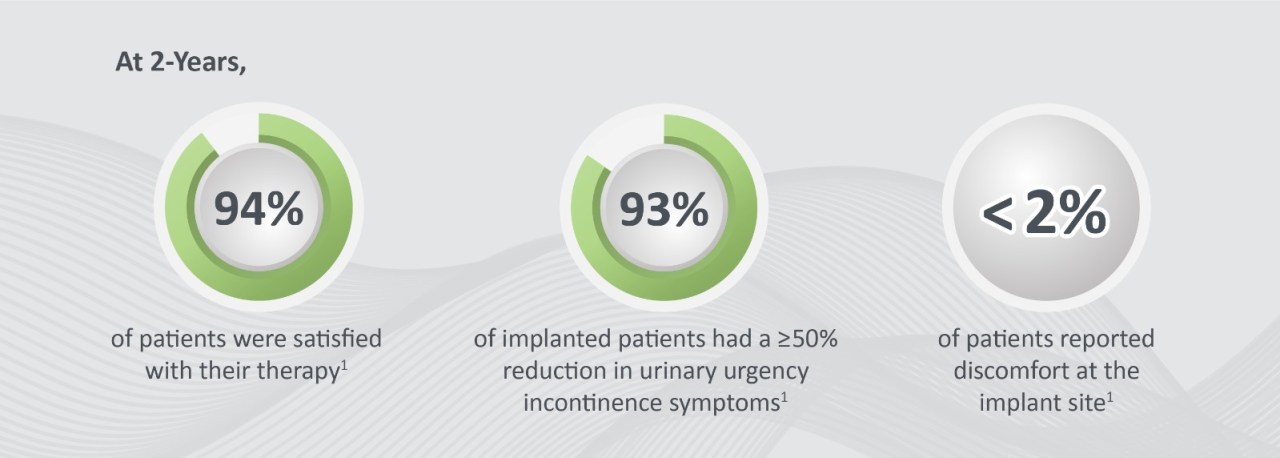
At 2-Years,
- 94% of patients were satisfied with their therapy1
- 93% of patients experienced a ≥50% reduction in urinary urgency incontinence symptoms1
- <2% of patients reported discomfort at the implant site1
Disclaimer: Results and experiences may vary.
Who is a good candidate for Axonics Therapy?
It is up to you and your healthcare provider to determine if you are a good candidate for Axonics Therapy.
Axonics Therapy is an approved treatment for patients suffering with:
- Overactive bladder (OAB) – the urgent need to urinate which may result in frequent urination and/or incontinence (leakage) episodes
- Urinary frequency – the need to urinate 8 or more times a day, which may or may not be associated with urgency
- Urinary urgency incontinence (UUI) – the urgent need to urinate or trouble holding urine before making it to the restroom
- Fecal (bowel) incontinence – involuntary loss of stool which may or may not be associated with urgency
- Nonobstructive urinary retention (UR) – the inability to completely empty the bladder which may result in trouble urinating or frequent small voids
- Axonics Therapy is indicated for patients who have failed conservative treatments, such as lifestyle changes, physical therapy, or medications
Schedule a Consultation in San Antonio, TX Today
Ready to take the first step towards symptom relief? Talk with our board-certified urologists and call (210) 267-1709 to schedule a consultation at our urology clinic in San Antonio, TX.
Important Safety Information:
Implantation and use of the Axonics System incurs risk beyond those normally associated with surgery, some of which may necessitate surgical intervention.
Results and experiences may vary and are unique to each patient. No promise or guarantee is made about specific results or experiences. Talk to your doctor about whether the Axonics System is right for you and to discuss the potential risks and benefits. For more information about safety and potential risks, go to: www.axonics.com/isi.
Caution: Federal law (USA) restricts this device to sale and use by, or on the order of, a physician.
1. Pezzella A, et al. Neurourol Urodyn. 2021
2. Blok, B, et al. Neurourol Urodyn. 2020)

 Small Size: Safely delivers therapy with a miniaturized implant
Small Size: Safely delivers therapy with a miniaturized implant